The Gwangju Institute of Science and Technology (GIST, Acting President Park Rae-gil) announced on the twenty eighth that a team led by Professor Kwangseop Eom of the Department of Materials Science and Engineering developed a technology that significantly improves the capability retention rate and sturdiness of lithium metal batteries through electrochemical pretreatment of copper current collectors.
The research team identified for the primary time the catalytic effect of lithium nitrate (LiNO3) decomposition of thiourea in an organic electrolyte, and thru a straightforward electrochemical process using this, an inorganic-rich artificial solid on the surface of a copper current collector utilized in a lithium metal battery anode. formed a barrier.
The strong physical properties and ionic conductivity of the substitute solid film have the effect of suppressing the expansion of lithium dendrites, which degrades the performance and sturdiness of the battery. It was confirmed that this was resulting from a considerable amount of inorganic matter resulting from the catalytic decomposition of lithium nitrate.
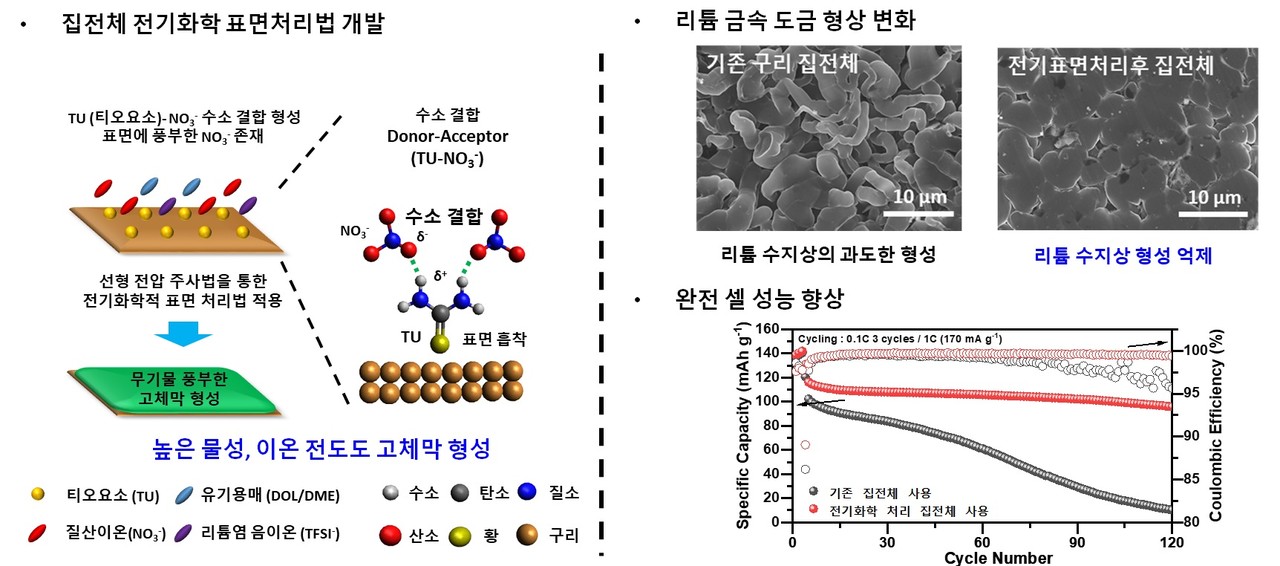
Because of this of using a lithium metal negative electrode using the developed copper current collector, the research team succeeded in manufacturing a lithium metal battery with a capability retention rate that’s about 2.5 times higher than that of the present copper current collector and a lifespan that’s greater than 4 times higher.
The prevailing copper current collector’s capability decreased to lower than 70% after about 30 charge/discharge cycles, however the anode using the newly developed copper current collector showed stable performance, maintaining its capability over 70% even after 120 charge/discharge cycles. .
Specifically, because the electrochemical treatment is performed only by applying a straightforward electrical signal akin to a voltage scan, it is feasible to simplify the electrode manufacturing process.
Professor Eom Kwang-seop said, “This research achievement is of great significance in that it has secured sufficient stability for use as a current collector for a negative electrode of a lithium metal battery with a small amount of electrolyte additives and straightforward electrochemical surface treatment.” We expect it to contribute to the commercialization of electrical vehicles equipped with
Reporter Hojeong Na hojeong9983@aitimes.com
